Establishing a robust FtsZ based divisome for synthetic cell division
Joris Dommisse
Project Summary
The ultimate goal of bottom-up biology is to create a living cell from non-living (biological) matter. The definition of such a synthetic, living cell can be interpreted quite broad, but for the purposes of this project, it is defined as: a membrane-bound aqueous compartment, that can sustain itself in a non-equilibrium state, possesses a genome (or similar) encoding its components, is able to autonomously grow and divide into identical daughter cells, that senses and responds to its environment, and that is able to evolve across generations. Realistically, such a huge scientific endeavor can only be accomplished by collaboration of many labs, which is exactly the point of the EVOLF project.
As part of EVOLF, my project focuses on generating constricted liposomes, mimicking the final stages of cell division. For this, we draw inspiration from the divisome of the well-studied bacteria Escherichia coli.
In vivo, E. coli division starts with formation of the Z-ring at the midplane of the cell. The Z-ring consists of FtsZ filaments, anchored to the membrane via anchor proteins (FtsA, ZipA). It is positioned at the midplane via oscillations of the MinCDE system. Once formed, the Z-ring acts as a scaffold for recruitment of further divisome proteins. Once matured, the Z-ring contracts in concert with peptidoglycan synthesis, resulting in septum formation and in the end division.
In vitro, successful constriction of giant liposomes with a Z-ring has been achieved using FtsZ and FtsA expressed with cell-free expression systems. However, the mechanism by which FtsZ can constrict the membrane - as well as the required conditions - are largely unknown. The aims of this project are therefore as follows:
-
To test FtsZ from different species for their ability to constrict liposomes, informed by bioinformatic analysis.
-
To determine optimal conditions for FtsZ-mediated liposome constriction, aided by machine learning.
-
To study the mechanism of force generation by FtsZ (in combination with FtsA).
-
And finally, to integrate constriction with other functions of the synthetic cell: chromosome segregation and abscission.
To accomplish these goals, I will use a combination of protein purification and reconstitution, cell-free expression, GUV production techniques, microfluidics, light-microscopy, image flow cytometry and machine-learning assisted methods.
I have a joint position between Koenderink Lab and Dogterom Lab.
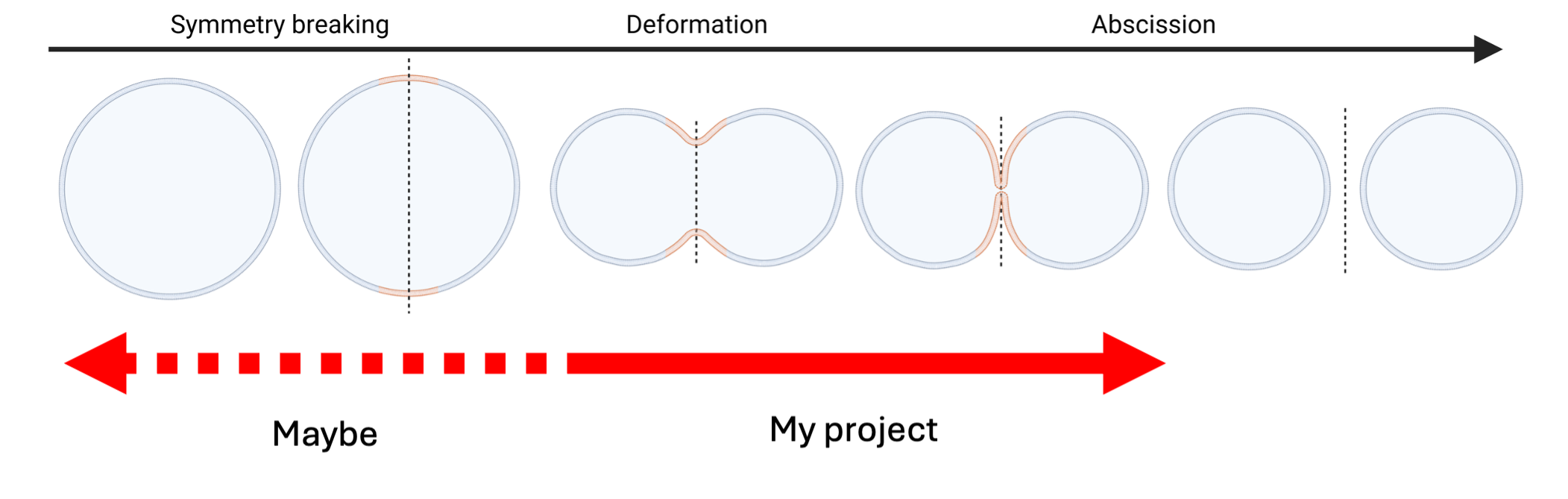
Figure 1 Schematic overview of the steps for division. The FtsZ system has been demonstrated to induce deformation of liposomes and constrict them, potentially it could also lead to equally sized daughters upon constriction by itself.
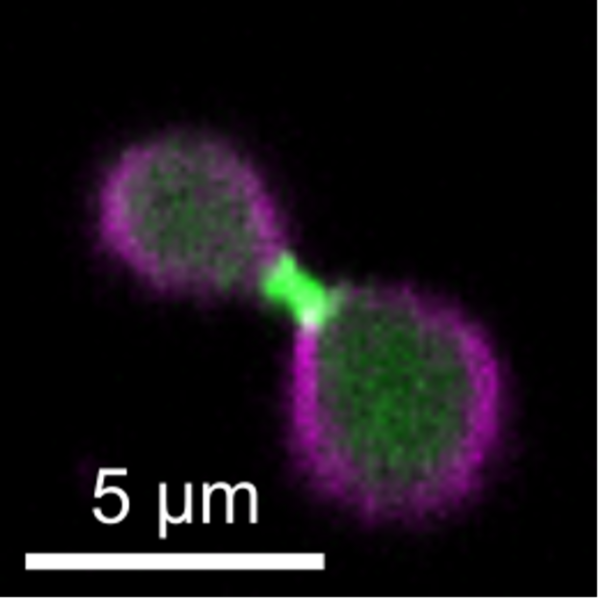
Figure 1 Confocal image of a liposome (magenta) constricted by cell-free expressed FtsZ (green) and FtsA (not labeled).





